Extraglycemic Outcomes and AEs
Patients with diabetes are at increased risk for developing macrovascular and microvascular complications, leading to physical disability, psychological distress, and overwhelming healthcare costs. The control of modifiable risk factors, such as hyperglycemia, obesity, hypertension, albuminuria, dyslipidemia, and smoking has significantly reduced the burden of diabetes. The widespread use of effective antidiabetic agents, statins, platelet inhibitors, and renin-angiotensin-aldosterone system (RAAS) inhibitors has decreased the risk of type 2 diabetes-related myocardial infarction, stroke, amputations, and mortality by >50%.1
GLP-1 receptor agonists have several added benefits in addition to effectively lowering Ab1c. Some GLP-1 receptor agonists have demonstrated a reduction in major adverse cardiovascular events, such as non-fatal myocardial infarction, non-fatal stroke, and cardiovascular death, in patients with type 2 diabetes and established atherosclerotic cardiovascular disease.2-7 There is some evidence to suggest that GLP-1 receptor agonists may also decrease the risk of kidney disease.8
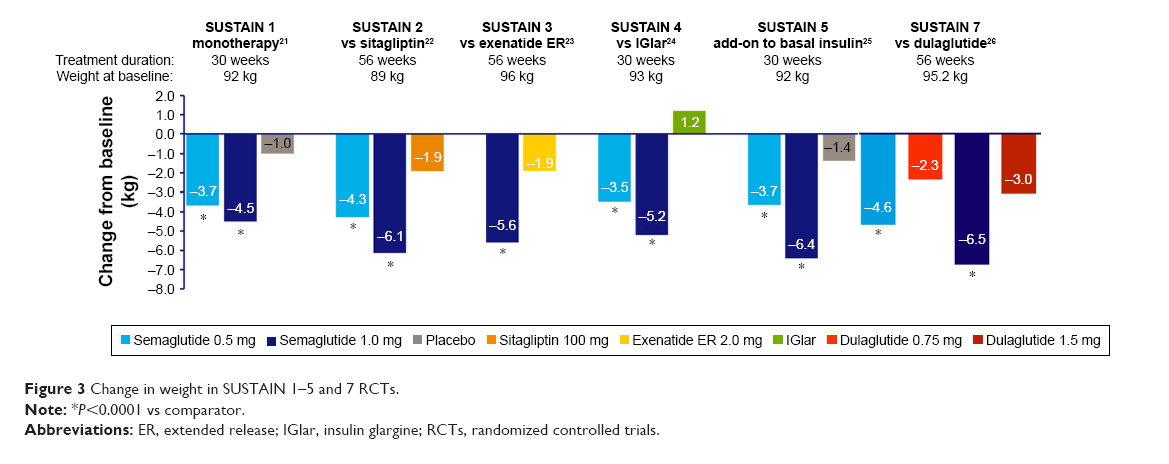
GLP-1 receptor agonists are also associated with significant weight loss. A meta-analysis found that GLP-1 receptor agonists lead to an average weight loss of 0.79-1.38 kg compared with placebo, and a loss of 1.00-7.30 kg compared to antidiabetics drugs that are associated with weight gain, such as insulin, sulfonylureas, and thiazolidinediones.9,10Once daily liraglutide 3.0 mg, and once weekly semaglutide 2.4 mg, have been FDA-approved for chronic weight management providing a novel effective strategy against obesity. The mean weight loss difference between GLP-1 receptor agonists and placebo as add-on to lifestyle intervention in patients with diabetes was 4% to 6.2% compared to 6.1 to 17.4% in people without diabetes.11 Weight loss with these agents is likely the result of GLP-1-mediated reduction in appetite and food intake. Figures 1 and 2 compare the average weight loss of participants in several clinical trials of GLP-1 receptor agonists.
Figure 1: Average Weight Loss in Randomized, Head-to-Head Clinical Trials of GLP-1 Receptor Agonists in Type 2 Diabetes12
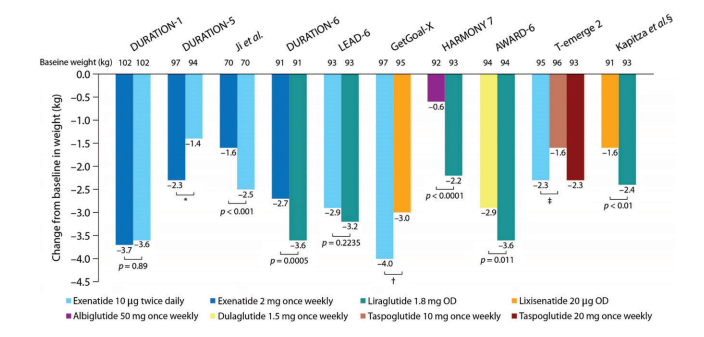
Figure 2: Average Weight Loss in Select Phase III Trials of GLP-1 Receptor Agonists13
References:
- Muskiet MHA, Tonneijck L, Smits MM, et al. GLP-1 and the kidney: From physiology to pharmacology and outcomes in diabetes. Nat Rev Nephrol. 2017;13:605-628.
- Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247-2257.
- Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228-1239.
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double–blind, randomized placebo–controlled trial. Lancet. 2019;394:121-130.
- Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841-851.
- Mosenzon O, Schechter M, Leibowitz G. Kidney outcomes with glucagon–like peptide–1 receptor agonists in patients with type 2 diabetes. Adv Chronic Kidney Dis. 2021;28:347-360.
- Sun F, Chai S, Yu K, et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss in patients with type 2 diabetes: A systematic review and network meta-analysis. J Diabetes Res. 2015,157-201.
- Liu,SC, Tu YK, Chien MN, et al. Effect of antidiabetic agents added to metformin on glycaemic control, hypoglycaemia and weight change in patients with type 2 diabetes: A network meta-analysis. Diabetes Obes Metab. 2012;14:810-820.
- Jensterle M, Rizzo M, et al. Efficacy of GLP-1 RA approved for weight management in patients with or without diabetes: A narrative review. Adv Ther. 2022;39:2452-2467.
- Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: A review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2015;6:19-28.
- Gomez-Peralta F, Abreu C. Profile of semaglutide in the management of type 2 diabetes: Design, development, and place in therapy. Drug Des Devel Ther. 2019;13:731-738.
Adults with type 2 diabetes have a 2-to-4-fold higher risk of cardiovascular morbidity and mortality than adults without diabetes. Atherosclerotic cardiovascular disease (ASCVD) is defined as coronary heart disease, cerebrovascular disease, or peripheral arterial disease of atherosclerotic origin. ASCVD is the leading cause of morbidity and mortality in type 2 diabetes and is the largest contributor to both direct and indirect diabetes costs. Diabetes-related risk factors for ASCVD include dyslipidemia, hypertension, hyperglycemia and insulin resistance. These diseases also share common lifestyle risk factors, such as obesity, unhealthy diet, sedentary lifestyle, and smoking.1 In a study of 530,747 adults with type 2 diabetes in the US, only 6.4% did not have any other cardiovascular, renal, or metabolic conditions. Over half of the patients in the study had 3 or more other conditions in addition to diabetes. The most prevalent individual conditions were hypertension (83%), hyperlipidemia (81%), coronary artery disease (32%) and chronic kidney disease (CKD) (20%), and the most common combinations included various groupings of hypertension, hyperlipidemia, atherosclerotic cardiovascular disease and CKD.2
Several diabetes-related biological mechanisms related to high blood glucose, including increased oxidative stress, increased coagulability, endothelial dysfunction and autonomic neuropathy, increase the risk of CVD in patients with T2DM.3 Observations of the excess prevalence and severity of CVD in patients with T2DM made the reduction of blood glucose a logical target for improving CV outcomes. However, large randomized control trials, such as ADVANCE and ACCORD showed that glycemic interventions alone are insufficient to reduce CV risk. In recent years, an array of strategies for reducing CV risk in patients with T2DM has become available, including advances in anticoagulation and antiplatelet management, novel lipid modifying therapies, and the introduction of two classes of antihyperglycemic drugs, which decrease the risk of CV events: GLP-1 RAs and sodium-glucose cotransporter-2 (SGLT2) inhibitors. The CV benefits of these agents result from mechanisms that are independent of their glucose-lowering effects.4 The ADA’s Standards of Medical Care in Diabetes 2023 recommends that the decision to treat high-risk individuals with a GLP-1 receptor agonist or SGLT2 inhibitor to reduce major adverse cardiovascular events (MACE), hospitalization for heart failure (hHF), cardiovascular death, or chronic kidney disease (CKD) progression should be considered independently of baseline HbA1c or individualized HbA1c target. GLP-1 receptor agonists can also be considered in patients with type 2 diabetes without established cardiovascular disease (CVD). SGLT2 inhibitors are recommended in patients with type 2 diabetes and heart failure, particularly those with heart failure with reduced ejection fraction, to reduce hHF, MACE, and CVD death. They are also recommended in patients with type 2 diabetes with CKD to prevent the progression of CKD, hHF, MACE, and cardiovascular death.5 These recommendations were made on the basis of the results from FDA-mandated CV outcomes trials (CVOT), in which manufacturers were directed to show that new diabetes treatments did not result in an unacceptable risk of CV harms. These trials unexpectedly demonstrated that GLP-1 RAs and SGLT2 inhibitors are cardioprotective in patients with T2DM, with SGLT2 inhibitors primarily preventing heart failure, GLP-1 RAs primarily preventing atherosclerotic events, and both potentially providing renal protection.3
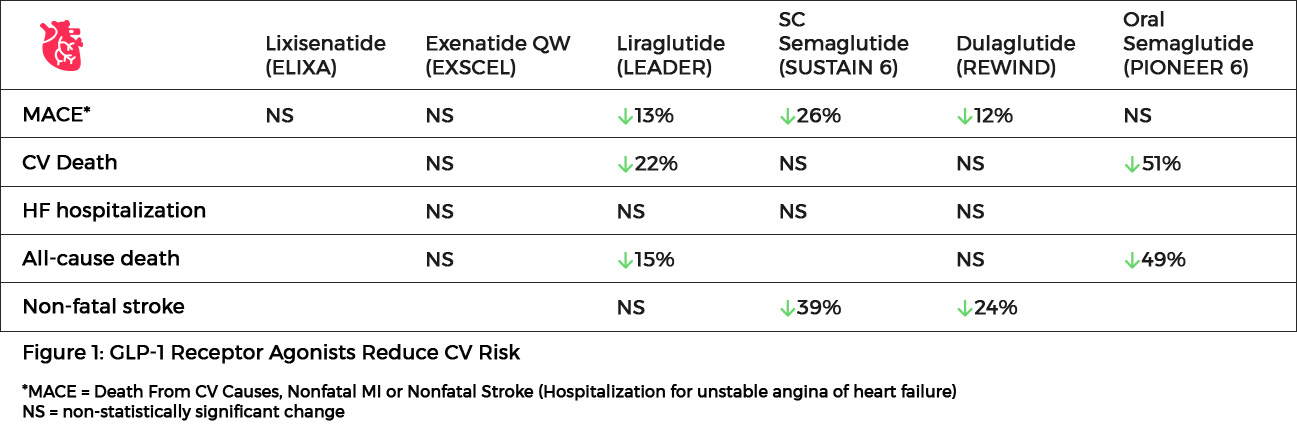
- In the SUSTAIN-6 study of 3297 patients with type 2 diabetes and high CV risk, the rate of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke was significantly lower among patients receiving semaglutide compared to placebo (6.6% vs 8.9%; HR, 0.74; P<0.001 for noninferiority). Semaglutide use was associated with a reduction in nonfatal myocardial infarction (2.9% vs 3.9%; HR, 0.74; P=0.12) and nonfatal stroke (1.6% vs 2.7%; HR, 0.61; P=0.04) compared to placebo. Rates of death from cardiovascular causes were similar between the two groups. At baseline, 83% of patients in the study had established cardiovascular disease, chronic kidney disease, or both.6
- The PIONEER 6 trial assessed cardiovascular outcomes of oral semaglutide compared to placebo in 3183 patients with type 2 diabetes at high CV risk. Major adverse cardiovascular events occurred in 3.8% of the semaglutide group and 4.8% of the placebo group (HR, 0.79; P<0.001 for noninferiority). A significant 51% relative reduction in the risk of cardiovascular death and a 49% relative reduction in all-cause mortality was noted with semaglutide use compared to placebo.7
- In the LEADER trial of 9340 patients with type 2 diabetes and high CV risk, significantly fewer patients who received liraglutide compared to placebo died from CV causes (4.7% vs 6.0%; HR, 0.78; P=0.007). The rate of death from any cause was also significantly lower in the liraglutide group than in the placebo group (8.2% vs 9.6%; HR, 0.85; P=0.02).8
- The REWIND trial investigated the effect of dulaglutide on major adverse cardiovascular events in 9901 patients with and without previous CV disease. The primary outcome of the first occurrence of the composite endpoint of non-fatal MI, non-fatal stroke, or death from CV causes occurred in significantly fewer patients receiving dulaglutide compared to placebo (12.0% vs 13.4%; HR, 0.88; P=0.026). All-cause mortality did not differ between the two treatment arms (10.8% for dulaglutide, 12.0% for placebo; HR, 0.90; P=0.067).9
- Several other GLP-1 receptor agonists have failed to show CV benefit in patients with type 2 diabetes. In the ELIXA trial of patients with type 2 diabetes and acute coronary syndrome, the addition of lixisenatide to usual care did not reduce the rate of major CV events (13.4% vs 13.2%) or rate of hospitalization for heart failure (HR, 0.96) compared to placebo.10 The EXSCEL study group found that once-weekly exenatide did not reduce the incidence of major CV events compared to those who received placebo in 14,752 type 2 diabetes patients (11.4% vs 12.2%; HR, 0.91).11 In the PIONEER 6 study, oral semaglutide met the primary outcome of non-inferiority and showed numerically favorable but not statistically significant results for reductions in 3-point MACE when compared with placebo (HR for oral semaglutide: 0.79; 95% CI: 0.57 to 1.11).7 PIONEER 6 had a relatively short duration of follow-up of just under 16 months and a relatively small study population of only 3,183 patients. Due to this study design the number of CV events was small, potentially accounting for the failure to reach statistical significance.12
- A meta-analysis of four large-scale cardiovascular outcome trials (SUSTAIN-6, LEADER, EXSCEL, and ELIXA) found a hazard ratio of 0.90 (95% CI 0.82-0.99; P=0.033) for treatment with a GLP-1 receptor agonist compared to placebo for major adverse cardiovascular events (cardiovascular mortality, non-fatal myocardial infarction, and non-fatal stroke).13
Figure 1: GLP-1 Receptor Agonists Reduce CV Risk6-12
References:
- ElSayed NA, Aleppo G, Aroda VR, et al. 10. Cardiovascular disease and risk management: Standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S158-S190.
- Arnold SV, Kosiborod M, Wang J, et al. Burden of cardio-renal-metabolic conditions in adults with type 2 diabetes within the Diabetes Collaborative Registry. Diabetes Obes Metab. 2018;20:2000-2003.
- Leon BM, Maddox TM. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6:1246-1258.
- Verma S, Mazer CD, Perkovic V. Is it time to REWIND the cardiorenal clock in diabetes? Lancet. 2019;394:95-97.
- ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: Standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S140-S157.
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
- Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841-851.
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): A double-blind, randomized placebo-controlled trial. Lancet. 2019;394:121-130.
- Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247-2257.
- Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228-1239.
- ADA 2019 Oral Semaglutide – The PIONEER Program Trials. touchENDOCRINOLOGY. Accessed January 20, 2023. https://www.touchendocrinology.com/insight/ada-2019-oral-semaglutide-the-pioneer-program-trials
- Bethel MA, Patel RA, Merrill P, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: A meta-analysis. Lancet Diabetes Endocrinol. 2018;6:105-113.
Although the use of pharmacologic agents and lifestyle modifications have reduced the risk of diabetic complications by >50%, the incidence of type 2 diabetes-related end-stage renal disease (ESRD) has only decreased by 29%. A persistently high number of patients with diabetes initiate renal replacement therapy (RRT) each year. Diabetes is the primary cause of chronic kidney disease (CKD) and ESRD in the world, accounting for approximately 33% of all patients initiating RRT. ESRD is a life-threatening complication resulting in a poor prognosis for patients with diabetes. Additionally, the combination of ESRD and diabetes leads to an increased risk of cardiovascular events.1,2A growing body of clinical and experimental studies suggests that GLP-1RAs may be associated with beneficial effects in patients with DKD independent of their glucose-lowering abilities, mediated by natriuresis, anti-inflammatory and anti-oxidative stress properties, and the suppression of renal fibrosis. Several trials have studied the association between GLP-1 receptor agonists and the development of diabetic kidney disease:
- In the SUSTAIN-6 trial of 3,297 patients with type 2 diabetes and cardiovascular disease (CVD) or significant CV risk factors, new or worsening nephropathy was assessed over a 2-year period in patients randomized to semaglutide or placebo. The primary kidney outcome was a composite of time to the development of new-onset persistent macroalbuminuria, persistent doubling of serum creatinine and eGFR <45 mL/min per 1.73m2, end-stage renal disease, and death due to CKD. Rates of new or worsening nephropathy were significantly lower with semaglutide (HR, 0.64; 95% CI, 0.46-0.88; P<0.01). This reduction was largely the result of a decrease in new-onset macroalbuminuria with semaglutide compared to placebo (2.5% vs 4.9%, respectively).3
- In the LEADER trial of 9,340 patients with type 2 diabetes at high risk for CVD, participants receiving liraglutide experienced a 22% reduction (P=0.003) compared to placebo in the same prespecified kidney outcome used in the SUSTAIN-6 trial. This benefit was driven by a 26% reduction in new-onset macroalbuminuria (HR, 0.74; 95% CI, 0.60-0.91; P=0.004).4
- In the LIRA-RENAL study, 279 patients with inadequately controlled type 2 diabetes and moderate renal impairment were randomized to once-daily liraglutide 1.8 mg or placebo. Liraglutide significantly reduced HbA1c from baseline (treatment difference, -0.66%; P<0.0001), decreased fasting plasma glucose (-1.22 mmol/L vs -0.57 mmol/L; P=0.036), and lowered body weight (-2.41 kg vs -1.09 kg; P = 0.0052). No changes in renal function were observed in either treatment group (eGFR relative ratio to baseline, -1% liraglutide vs +1% placebo; P=0.36).5
- In the REWIND study, which evaluated the cardiovascular safety of dulaglutide (1.5 mg) in comparison to placebo, the composite renal outcome (the first occurrence of new macroalbuminuria, a sustained decline in eGFR of ≥30% from baseline, or RRT) developed at a lower incidence rate in patients who received dulaglutide vs those who received placebo [HR 0.85 (95% CI: 0.77–0.93), p=0.0004]. This result was largely driven by a reduced incidence of macroalbuminuria [HR 0.77 (95% CI: 0.68–0.87),
P< 0.001].6 - A significant reduction in albuminuria was also noted in clinical trials of other GLP-1 receptor agonists. Exenatide was associated with a reduction in new-onset macroalbuminuria (2.8% vs 2.2%; P=0.03) in patient with type 2 diabetes, 70% of whom had preexisting CVD.7 A trial with lixisenatide reported a modest reduction in albuminuria in 6,068 patients with type 2 diabetes and recent admission for acute coronary syndrome.8 In a comparison of once-weekly dulaglutide with insulin glargine in 576 subjects with type 2 diabetes and stage 3 or 4 CKD, dulaglutide use was associated with a significant reduction in albuminuria and a slowing of GFR decline in patients with macroalbuminuria at baseline.9
The potential for GLP-1 receptor agonists to favorably affect renal risk in diabetes may translate into further improvements of clinical outcomes beyond glycemic control. Additional trials are needed to further explore the effect of GLP-1 receptor agonists on kidney disease. Current treatment guidelines recommend the use of GLP-1 receptor agonists with proven cardiovascular benefit in patients with chronic kidney disease if the patient is unable to tolerate SGLT2 inhibitors or if eGFR is less than adequate.10
References:
- Muskiet MHA, Tonneijck L, Smits MM, et al. GLP-1 and the kidney: From physiology to pharmacology and outcomes in diabetes. Nat Rev Nephrol. 2017;13:605-628.
- Narres M, Claessen H, Droste S, et al. The incidence of end-stage renal disease in the diabetic (compared to the non-diabetic) population: A systematic review. PLoS One. 2016;11:e0147329.
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
- Mann JFE, Orsted DD, Brown-Frandsen K, et al. Liraglutide and kidney outcomes in type 2 diabetes. N Engl J Med. 2017;377:839-848.
- Davies MJ, Bain SC, Atkin SL, et al. Efficacy and safety of liraglutide versus placebo as add-on to glucose-lowering therapy in patients with type 2 diabetes and moderate renal impairment (LIRA-RENAL): A randomized clinical trial. Diabetes Care. 2016;39:222-230.
- Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and renal outcomes in type 2 diabetes: An exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394:131-138.
- Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228-1239.
- Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247-2257.
- Tuttle KR, Lakshmanan MC, Gross JL, et al. Comparable glycaemic control with once-weekly dulaglutide vs insulin glargine, both combined with lispro, in type 2 diabetes and chronic kidney disease (AWARD-7). Diabetologia. 2017;60(suppl 1):S3.
- ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: Standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S140-S157.
The majority of adverse events experienced with GLP-1 receptor agonist therapy affect the gastrointestinal system. The most commonly reported adverse events are nausea, vomiting, diarrhea, and injection-site reactions.1 Although these adverse events are relatively common, these symptoms gradually decrease as therapy continues. Nausea is the most common adverse effect, reported in up to 50% of patients depending on the agent. Nausea is generally mild to moderate in intensity and appears to be dose-dependent. The delay in gastric emptying seen with short-acting GLP-1 receptor agonists has been linked to a greater occurrence of nausea.2
Hypoglycemia rates are low with GLP-1 receptor agonists due to the glucose-dependent mechanism of action of these agents. Hypoglycemia rates are similar between GLP-1 receptor agonist medications and primarily occur when used concomitantly with sulfonylureas.1
GLP-1 Receptor Agonists: A Comparison of hypoglycemia rates in head-to-head clinical trials1
| Study | Active comparators | Major hypoglycemia (No sulfonylurea) | Sulfonylurea | Minor hypoglycemia (No sulfonylurea) | Sulfonylurea |
|---|---|---|---|---|---|
| DURATION-1 | Exenatide 10 µg BID | 0% | 0% | 1.1% | 14.8% |
| Exenatide 2 mg QW | 0% | 0% | 0% | 14.5% | |
| LEAD-6 | Exenatide 10 µg BID | 0% | 1.2% | 11.1% | 42.0% |
| Liraglutide 1.8 mg QD | 0% | 0% | 6.2% | 32.7% | |
| DURATION-5 | Exenatide 10 µg BID | 0% | 0% | 0% | 11.8% |
| Exenatide 2 mg QW | 0% | 0% | 0% | 12.5% | |
| DURATION-6 | Exenatide 2 mg QW | 0% | 0% | 3.6% | 15.3% |
| Liraglutide 1.8 mg QD | 0% | 0% | 2.6% | 12.2% | |
| GetGoal-X | Lixisenatide 20 µg QD | 0% | 0% | 2.5% | – |
| Exenatide 10 µg BID | 0% | 0% | 7.9% | – | |
| HARMONY-7 | Albiglutide 50 mg QW | 0% | 0% | 2.4% | 15.9% |
| Liraglutide 1.8 mg QD | 0% | 0% | 4.5% | 19.3% | |
| AWARD-1 | Dulaglutide 1.5 mg QW | 0% | 0% | 10.4% | – |
| Dulaglutide 0.75 mg QW | 0% | 0% | 10.7% | – | |
| Exenatide 10 µg BID | 0% | 0% | 15.9% | – | |
| Placebo | 0% | 0% | 3.5% | – | |
| AWARD-6 | Dulaglutide 1.5 mg QW | 0% | 0% | 8.7% | – |
| Liraglutide 1.8m g QD | 0% | 0% | 5.7% | – |
Prescribing information for GLP-1 receptor agonists warn of an increased risk of pancreatitis. In 2008, a report was published identifying 30 cases of acute pancreatitis associated with exenatide use. A review of reported adverse events in the US Food and Drug Administration’s database between 2004-2009 found the use of exenatide increased the odds ratio for reported pancreatitis by 6-fold compared to 4 other control medications (rosiglitazone, repaglinide, nateglinide, and glipizide). There was also a significantly greater risk of pancreatic cancer in patients receiving exenatide.3
In a meta-analysis of 113 randomized clinical trials with a duration greater than 11 weeks, use of GLP-1 receptor agonists was not associated with a higher incidence of pancreatitis or pancreatic cancer compared to comparator arms (OR, 0.93 vs 0.94, respectively). Of the trials included in the meta-analysis, 13 did not report pancreatitis rates and 72 did not report any pancreatic events occurring in any treatment group.4 A second meta-analysis investigated the risk of acute pancreatitis in long-term, placebo-controlled trials of GLP-1 receptor agonists that had a duration greater than 24 months. Three high-quality randomized controlled trials enrolling a total of 9,347 GLP-1 receptor agonist-treated and 9,353 placebo-treated participants were included in the meta-analysis. GLP-1 receptor agonists were not associated with an increased risk of acute pancreatitis in this study.5 Additional long-term studies will need to be conducted to confirm an association between GLP-1 receptor agonist use and pancreatitis.
Preclinical studies in mice and rats have shown that stimulating the GLP-1 receptor raises cAMP in thyroid C cells and initiates the release of calcitonin. In rodents, long-term exposure to GLP-1 receptor agonists is accompanied by C-cell proliferation and the formation of medullary thyroid carcinomas. It has been hypothesized that thyroid tumors are more common in rodents because the expression of GLP-1 receptors on thyroid C cells is far greater in rodents than in humans.6 A meta-analysis of 25 studies showed that liraglutide was not significantly associated with an increased risk of thyroid cancer (OR, 1.54) and no thyroid malignancies were reported with exenatide.7 More information is needed to reach a consensus on the risk of thyroid cancer in humans who use GLP-1 receptor agonists. GLP-1 receptor agonists are not recommended in patients with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2.6
References:
- Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: A review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2015;6:19-28.
- Filippatos TD, Panagiotopoulou TV, Elisaf MS. Adverse effects of GLP-1 receptor agonists. Rev Diabet Stud. 2014;11:202-230.
- Elashoff M, Matveyenko AV, Gier B, et al. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141:150-156.
- Monami M, Nreu B, Scatena A, et al. Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): Data from randomized controlled trials. Diabetes Obes Metab. 2017;19:1233-1241.
- Storgaard H, Cold F, Gluud LL, et al. Glucagon-like peptide-1 receptor agonists and risk of acute pancreatitis in patients with type 2 diabetes. Diabetes Obes Metab. 2017;19:906-908.
- Nauck MA, Friedrich N. Do GLP-1-based therapies increase cancer risk? Diabetes Care. 2013;36:S245-S252.
- Alves C, Batel-Marques F, Macedo AF. A meta-analysis of serious adverse events reported with exenatide and liraglutide: Acute pancreatitis and cancer. Diabetes Res Clin Pract. 2012;98:271-284.